12 Best Images of Acid Rain And Ph Worksheet Answers AcidBase Chemistry Worksheets, Acids and
A weak acid is a hydrogen-bearing molecular compound that ionizes only slightly in water solution. Using HX (aq) as the formula of a weak acid dissolved in water, the ionization equilibrium is. HX (aq) H + (aq) + X- (aq) (1.7) H 2 O (l) + HX (aq) H 3 O + (aq) + X - (aq) and the associated equilibrium expression is.

Acids and Bases Worksheet Yr 9 Science
This free acid and bases worksheet pdf contains. Acid and Base Sort Challenge: It's like a science puzzle! Students will get to play detective as they cut and paste common household acids and bases, sorting them into their acidic or basic homes. The Litmus Test (My Personal Favorite): Brace yourself for some mind-boggling fun!

Properties Of Acids And Bases Worksheet worksheet
Acids & alkalis for KS3 Science - Worksheet (Answers) 1. Colour the pH scale and add the following labels: Neutral, Strong acid, weak acid, strong alkali and weak alkali.. Alkalis are special types of bases, which property do alkalis have? Alkali are bases that dissolve in water 5. What are the names and chemical formula of three common acids

susan [46+] Acids And Bases Guided Answer Key, Acids And Bases Answer Key Bomtron
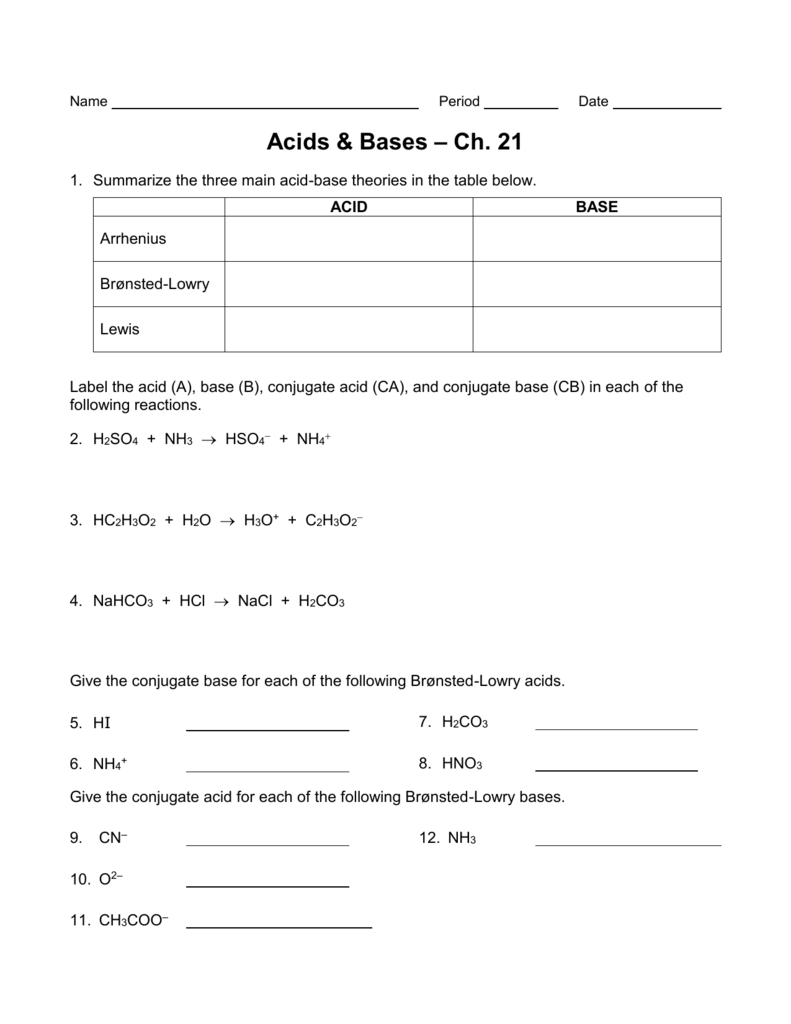
Brønsted-Lowry. The Brønsted-Lowry definition of acids and bases liberates the acid-base concept from its limitation to aqueous solutions, as well as the requirement that bases contain the hydroxyl group. A Brønsted-Lowry acid is a hydrogen-containing species which is capable of acting as a proton (hydrogen ion) donor. A Brønsted-Lowry base is a species which is capable of acting.
Intro to Acids & Bases Worksheet
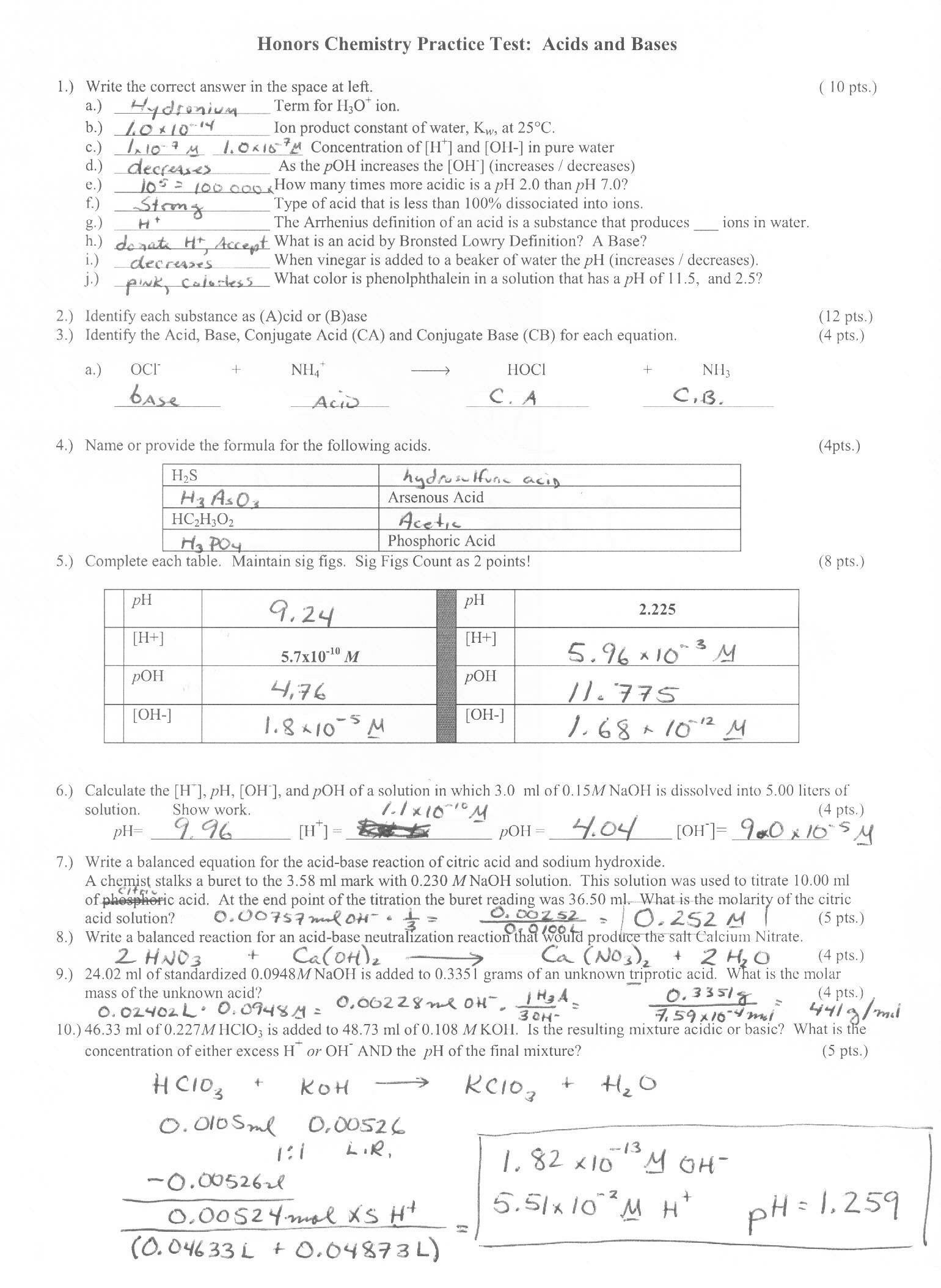
Naming Acids and Bases Answers Name the following acids and bases: 1) NaOH sodium hydroxide 2) H2SO 3 sulfurous acid 3) H2S hydrosulfuric acid 4) H3PO 4 phosphoric acid 5) NH 3 ammonia 6) HCN hydrocyanic acid 7) Ca(OH) 2 calcium hydroxide 8) Fe(OH) 3 iron (III) hydroxide 9) H3P hydrophosphoric acid Write the formulas of the following acids and.

Acids Bases Worksheet MHS Pre
10. Explain in terms of the partial charge on hydrogen why NaOH is a base, HClO is a weak acid and HClO 4 is a strong acid. 11. Why is HCl a strong acid and HClO a weak acid? 12. Why are HCl and HClO 4 both strong acids? 13. For each of the reactions below, classify the reactants as an acid or a base and the products as the conjugate acid or.

12 Best Images of Acid Rain And Ph Worksheet Answers AcidBase Chemistry Worksheets, Acids and
Acids and Bases Acids and Bases teachernela Member for 3 years 4 months Age: 11-15 Level: Grade 9 Language: English (en) ID: 357911 10/09/2020 Country code: PH Country: Philippines School subject: Science (1061951) Main content: Acids and Bases (1993250) Acids and Bases Other contents: Acids and Bases Loading ad. Share / Print Worksheet

Top 12 Acids And Bases Worksheet Templates free to download in PDF format
This page titled Acids and Bases (Worksheet) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Mark Draganjac via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.

Acids And Bases Worksheet printable pdf download
ACIDS AND BASES LESSON PLAN WORKSHEETS. The Acids and Bases lesson plan includes three worksheets: an activity worksheet, a practice worksheet, and a homework assignment. Each worksheet will help reinforce students' comprehension of the concepts and material they learned throughout the lesson. You can refer to the guide on the classroom.

GoodScienceWorksheets's Shop Teaching Resources TES
c) A 0.0010 M solution of a weak acid with K a = 5.0 x 10 -5. 8. Draw a series of illustrations that show what occurs at the particulate level (a) before solid sodium acetate and water are combined, (b) just after the sodium acetate is added to the water, and (c) after the solution comes to equilibrium. 9.

Acids and Bases Worksheet WordMint
Acids/Bases & pH Worksheet PROPERTIES OF ACIDS AND BASES 1. Acids taste _________________ and bases taste __________________, bases also feel 2. Acids turn litmus paper __________, bases turn litmus paper ___________ and phenolphthalein 3. Circle: Weak acids are strong / weak electrolytes.

Quiz & Worksheet Household Acids, Bases & Salts
What are acids and bases? Many common pure substances can be classified according to whether they are acids or bases. Acids produce hydrogen ions (H+) and bases produce hydroxide ions (OH-) when dissolved in solution. The concentration of hydrogen ions refers to the number of hydrogen ions in a specific volume of solution.

Acids And Bases Worksheet Ph Ph Practice Worksheet Acid and base
TASK 1 - Bronsted-Lowry acids & bases Identify the Bronsted-Lowry acid and base in each of the following reactions. SECTION 2 - pH of strong acids Number of protons released Monoprotic acid = acid that releases one H+ ion per molecule e.g. HCl (hydrochloric acid), HNO3 (nitric acid), CH3COOH (ethanoic acid) Diprotic acid =

Worksheet acids and bases
Acids and Bases Worksheets. This fantastic bundle includes everything you need to know about Acids and Bases across 24 in-depth pages. These ready-to-use worksheets are perfect for teaching kids about Acids and Bases. Acids and bases are two types of compounds. To some extent, almost all liquids are acids or bases.

Naming Acids Worksheet SexiezPicz Web Porn
Acids and bases worksheets for Grade 12 are an essential resource for teachers looking to enhance their students' understanding of these crucial concepts in Science and Chemistry. These worksheets provide a comprehensive and engaging way for students to practice their skills, solidify their knowledge, and prepare for exams..

Phet Acids And Bases Worksheet Phet Acid Base Worksheets Teaching Resources Tpt Fay Vipinted
Acids and bases are chemical substances that react with each other and may affect pH levels. Acids are characteristically sour, corrosive, and neutralized by bases. The definitions of acids and bases depend on the Arrhenius, Brønsted-Lowry, and Lewis theories.